Celluma Light Therapy Receives EU-MDR Certification Company expands product offerings in the UK, EU and Switzerland

Anaheim, CA – April 4, 2023 – BioPhotas, Inc., the manufacturer of the award-winning Celluma SERIES of light therapy devices, announced today that the Company received certification to European Council Medical Device Regulation 2017/745 for low level light therapy devices. Over the course of the last several years, the European Medical Device Authorities have been transitioning from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR). The Company believes it is the first low-level light therapy device manufacturer to be certified to the new MDR standards.
Speaking on this recent development, Patrick Johnson, Chief Executive Officer of BioPhotas said, “This certification represents a major milestone for BioPhotas and means that in addition to its legacy products, the Celluma RESTORE for hair restoration will be available in the European Union and other countries that rely on certification to the MDR. Demonstrating compliance to the MDR requirements has been a dauting task for medical device manufacturers around the world and after a multi-year effort, we are proud to say our products comply with the most rigorous regulatory requirements around the world.”
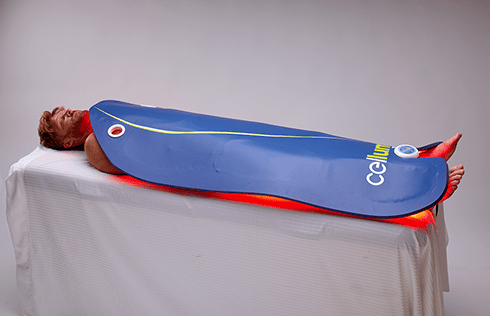
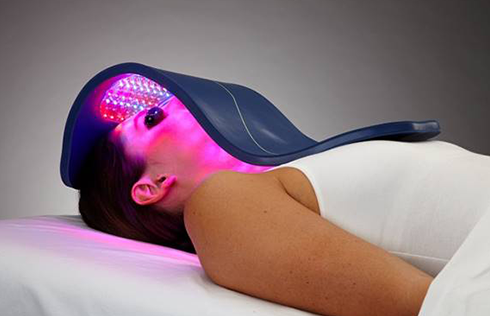
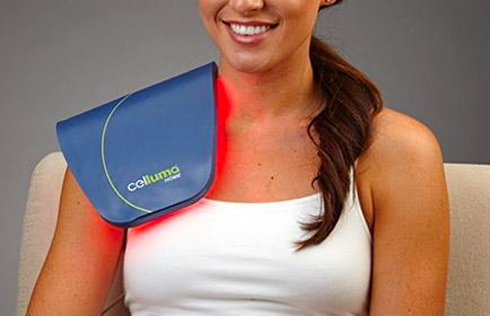
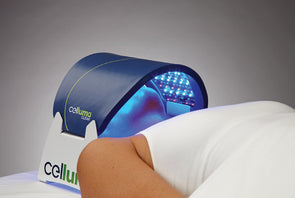
Rapidly approaching 100,000 light therapy panels sold around the globe, the Celluma SERIES continues to be recognized as best-in-class LED light therapy, amassing nearly 70 industry awards for best product, most innovative product and best new product in the medical aesthetics and pain management space. The company’s flagship product, the Celluma PRO, continues to reign as the most-awarded LED panel on the market and the Celluma DELUX is the first portable full-body device that carries multiple regulatory credentials and eliminates the exorbitant cost and space requirements of LED beds. The Celluma SERIES is legally registered for sale in 98 countries world-wide.
Commenting further, Mr. Johnson shared, “when we founded the Company 12 years ago, our fundamental objective was to build a best-in-class medical device company, manufacturing low level light therapy devices that conformed to the most stringent regulatory requirements. While we could have gone to market as a cosmetic company making soft claims about our products, we felt strongly that the performance of our products should be based on robust science and what we say about our products should be regulated by globally recognized requirements. It’s satisfying that we are living up to those founding objectives.”
As previously announced, the Company relocated into a new corporate headquarters in Tustin last month, a move made specifically to expand manufacturing capacity and support continued growth of domestic and international sales.