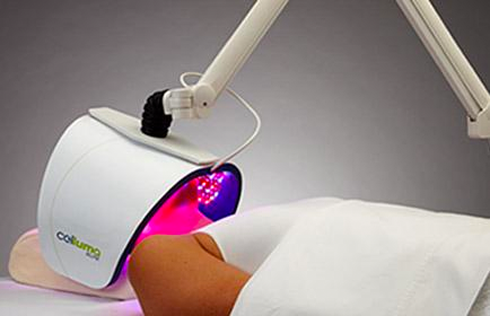
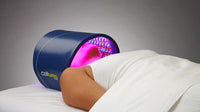
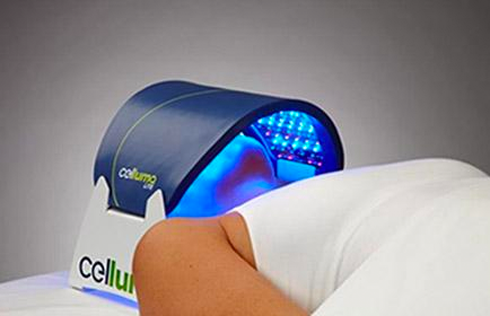
Celluma Launches New Hair Restoration LED Light Therapy Device

Anaheim, California – July 8, 2021 - BioPhotas, Inc., the market leader in therapeutic LED Light Therapy devices announced today a major product line extension to the Company’s award-winning Celluma SERIES of Light Therapy Devices. The all-in-one Celluma RESTORE is the first LED device FDA-cleared for over-the-counter (OTC) use to treat three common concerns of the mature individual in a single device: hair loss, aging skin, and general pain conditions. This is the 14th model in the Celluma SERIES.
Commenting on the latest addition to the product line, BioPhotas President and Chief Executive Officer Patrick Johnson said, “It’s great to add a Hair Restoration indication-for-use to our long list of treatment clearances from the FDA. Providing a single device that treats whole body aches and pains, fine lines and wrinkles, and hair loss on the scalp is the ultimate baby-boomer anti-aging device. Now the mature individual interested in feeling and looking great, and retaining their youthful appearance, can have a device at home that meets all of their rejuvenating needs.” The Celluma RESTORE is targeted directly at the multi-billion dollar global health and wellness market, specifically, the non-invasive hair restoration segment of the medical aesthetics industry.
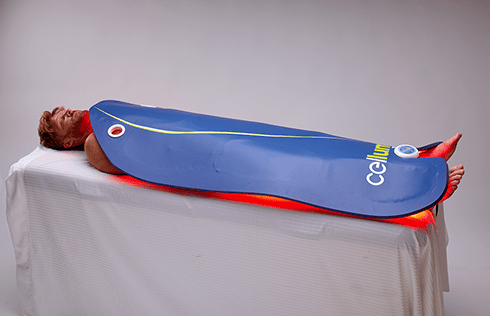
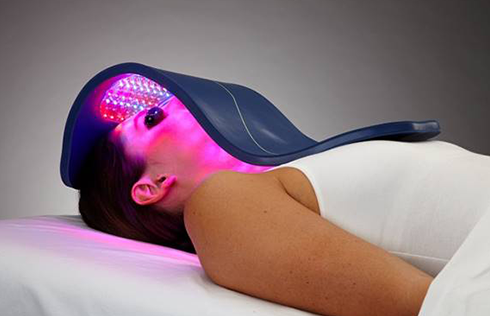
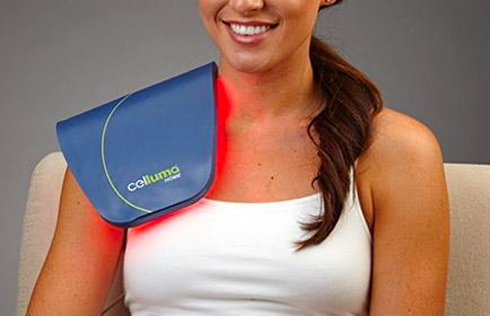
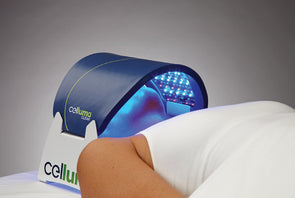
The Celluma RESTORE is non-toxic, non-invasive, and painless. The device is versatile, convenient, and easy-to-use in both a clinical setting or at home. Celluma’s patented, shape-taking design permits customized placement over the head to effectively deliver light energy and promote hair regrowth. Additionally, the Celluma RESTORE can be placed anywhere on the body to reduce pain and improve skin health. Measuring 16” x 8” overall with a treatment area of 14” x 6”, the Celluma RESTORE can be stored flat in a drawer for convenience and easy access.
Commenting further on the announcement, Mr. Johnson said, “Having just celebrated the 10th anniversary of BioPhotas, it is only appropriate that we receive our 10th indication-for-use clearance from the FDA. The Celluma SERIES now has more regulatory credentials than any other LED device in the world and is legally registered for sale in 78 countries around the globe. We anticipate that the new RESTORE will be available to our U.S. customers in mid-August. The new device is already slated for MDR Tech File review later this summer and should be available in the E.U. and U.K. in the 4th quarter.”
The Celluma SERIES has also continued its winning ways by adding five new industry awards in the first half of 2021. Celluma Light Therapy was recognized by Les Nouvelles Esthetiques & Spa Magazine with two Best Product awards. Dermascope Magazine also recognized Celluma devices with two Aesthetician’s Choice Awards. And the readers of Skin Deep Magazine awarded Celluma the Reader’s Choice award for Favorite LED Device, making the Celluma SERIES the most award-winning LED devices with more than 40 international awards.
Visit www.celluma.com to see the complete line of Celluma devices.
About BioPhotas, Inc.
In providing unique devices founded on NASA-developed technology and backed by clinical studies, BioPhotas is bringing to market safe, effective and affordable devices that unlock the clinical power of biophotonics. BioPhotas develops and markets devices for healthcare providers and consumers that conveniently treat a variety of skin, muscle and joint conditions.