BioPhotas Inc. Announces Product Line Expansion and Sales Office in London, England, for 2019

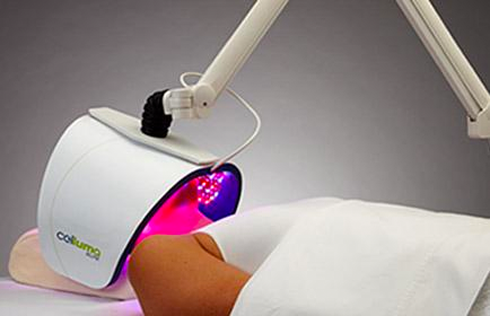
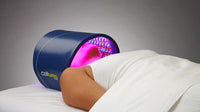
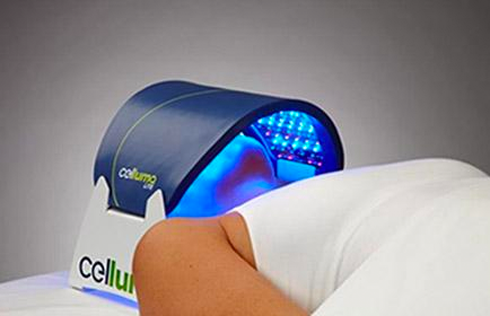
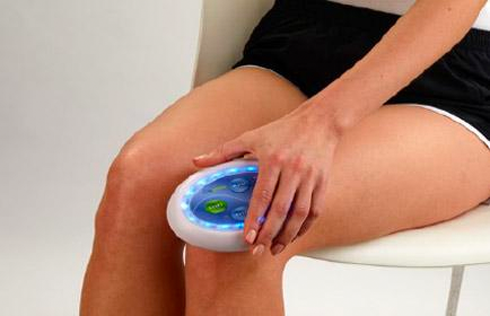
Anaheim, California – January 2, 2019 - BioPhotas, Inc., the market leader in therapeutic LED Light Therapy devices announced today a major product line extension to the Company’s Celluma Series of Light Therapy Devices. In addition, the Company is now offering a 2-year manufacturer’s warranty on all new product sales.
Commenting on these developments, BioPhotas’ President and CEO, Patrick Johnson said “The Celluma Series of Light Therapy devices are the most versatile LED products available worldwide, offering a wide variety of clinical applications in a single product platform. Simply put, the Celluma provides clinical professionals as well as end-users, the biggest bang for the buck. That said, we understand that our customers are sometimes very focused in their practices, wanting products that are specifically tailored to a particular clinical specialty and/or condition. This product line expansion is intended to address this desire for condition specific device applications.”
For 2019, four new versions of the Celluma are being added to the Company’s product offering. This includes devices intended for professionals and consumers who are only interested in aesthetic treatment, professionals and consumers who are interested in just pain management and finally, anyone who wants to manage acne breakouts, whether they be teens or adults suffering from the condition. Mr. Johnson continued, “based on our commitment to continually improve the design and manufacture of the Celluma Series of products, we are adding an additional year to our standard manufacturer’s warranty . . . a reflection on the robust performance of the tens of thousands of devices we have sold in more than 70 countries around the world.”
The Company also announced the opening of sales offices in London, England and the launch of a new website. Commenting further, Mr. Johnson said “we see the international markets are ripe for growth, particularly in Europe, given that the Celluma Series is Medical CE-marked and the only LED product of its kind approved by the European Medical Device Authorities to be used as a Dermal Wound Healing device. The UK is a huge part of the European market and regardless of what happens with Brexit, we believe there is a huge benefit from us having a local presence in the UK.” BioPhotas intends to serve the Republic of Ireland and Northern Ireland out of its new London office.
Having been recognized with numerous Product of the Year and Company of Year awards by US-based and International trade publications in 2018, the Company is proud to have been notified recently of three similar awards for 2019, awards that will be formally announced in the coming months.
About BioPhotas, Inc.
In providing unique devices founded on NASA-developed technology and backed by clinical studies, BioPhotas is bringing to market safe, effective and affordable devices that unlock the clinical power of biophotonics. BioPhotas develops and markets devices for healthcare providers and consumers that conveniently treat a variety of skin, muscle and joint conditions.
Contact:
Patrick Johnson – CEO
BioPhotas, Inc.
patrick.johnson@biophotas.com