Celluma Light Therapy to Celebrate First Annual National Celluma Light Therapy Day on June 20th
Anaheim, CA – February 16, 2023 – BioPhotas, Inc., the manufacturer of the award-winning Celluma SERIES of light therapy devices, announced today that it is moving its corporate headquarters and manufacturing operations from Anaheim to Tustin, California. With the rapid growth in sales over the last several years, it was necessary to find a larger home for Celluma Light Therapy. The new headquarters boasts 25,000 square feet of office and warehouse space, facilitating continued growth throughout 2023 and beyond. This added space allows BioPhotas to increase production and streamline shipping operations related to increased international sales.
Speaking on these recent developments, Patrick Johnson, Chief Executive Officer of BioPhotas said, “we are very excited to be moving into a new corporate headquarters that is two and a half times larger than our current facilities. This provides the capacity needed to continue executing on our growth plans and provides for accelerating scale anticipated over the next five years. The benefits of light therapy in a number of explosive consumer segments including anti-aging, hair restoration and pain management are hitting a consumer awareness tipping point and Celluma is positioned at the forefront as the LED light therapy innovation leader. It is also especially gratifying to be back in the City of Tustin where the company was founded 12 years ago.”
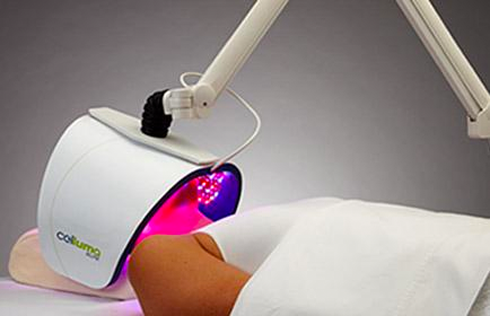
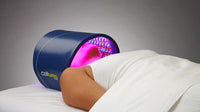
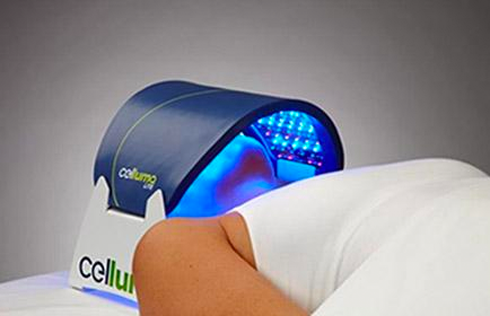
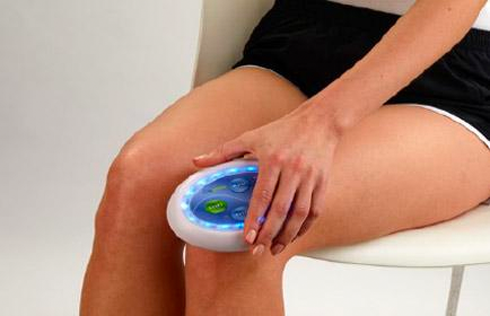
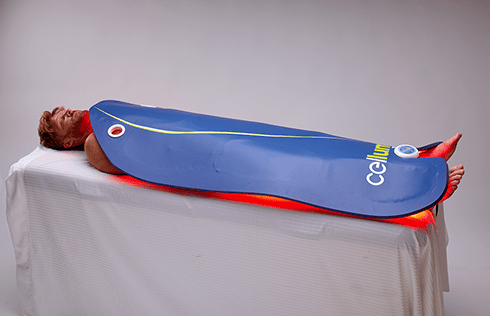
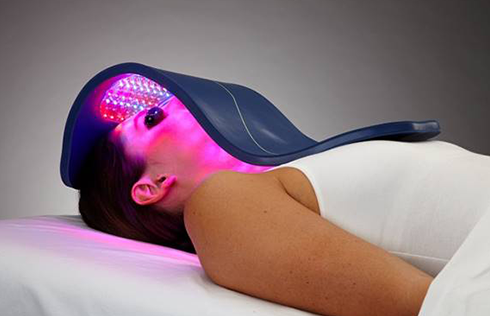
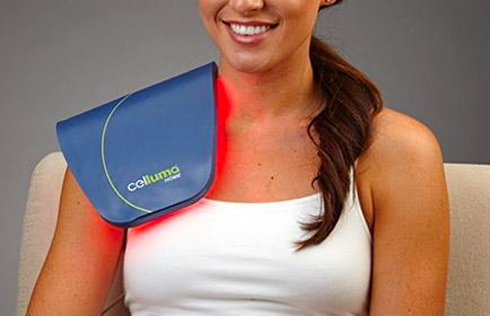
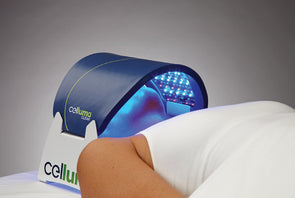
Rapidly approaching 100,000 light panels sold throughout the world, the Celluma SERIES continues to be recognized as best-in-class LED light therapy by both Dermascope Magazine and Les Nouvelles Esthetiques & Spa Magazine in 2023. The company’s flagship product, the Celluma PRO, continues to rein as the most awarded LED panel on the market and the Celluma DELUX was the Aesthetician’s Choice for best full-body light therapy device in 2023. The Celluma DELUX is the first portable full body device that carries multiple regulatory credentials and eliminates the exorbitant cost and space requirements of LED beds.
Commenting further, Mr. Johnson shared, “we are proud of the fact that the Celluma SERIES of light therapy devices are manufactured here in Orange County and the surrounding area. Not only have we contributed to the local economy through growing the size of our own workforce, but several of our key suppliers have grown along with us over the last twelve years, compounding the favorable impact we have had on the local medical device manufacturing eco-system. And because we manufacture locally, we have been fairly insulated from the supply chain issues that others have struggled with the last several years.”
This year has also seen a continued expansion of the Company’s international distribution network, with the addition of channel partners in Peru, Croatia, Denmark, Sweden, Finland, and Iceland. Used professionally or at-home, the Celluma SERIES of light therapy devices are now legally registered for sale on an over-the-counter or at-home basis in 98 countries around the world with additional expansion expected throughout 2023. The Company will be fully operational in their new facilities on March 13, 2023.